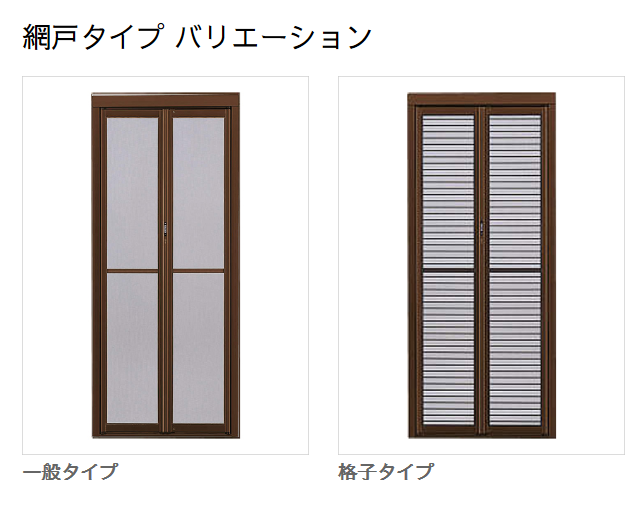
YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅805mm×高2300mm] ykk 玄関網戸 勝手口網戸 ドア用網戸 玄関引き戸用網戸 格子 折れ網
(税込) 送料込み
商品の説明
商品情報
【送料無料】取付、取り外しはネジを調整するだけ。ドライバー1本でOKです。幅+40ミリ・高さ+35ミリの調整幅がございます。【ykk】【玄関網戸】【勝手口網戸】【ドア用網戸】【玄関引き戸用網戸】【格子】【折れ網戸】
38660円YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅805mm×高2300mm] ykk 玄関網戸 勝手口網戸 ドア用網戸 玄関引き戸用網戸 格子 折れ網DIY、工具住宅設備中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社"https://item-shopping.c.yimg.jp/i/l/hokusei_nhm-08180-b","https://tshop.r10s.jp/hokusei-nw/cabinet/271m-so.jpg","https://tshop.r10s.jp/hokusei-nw/cabinet/267m-so.jpg","https://shopping.c.yimg.jp/lib/hokusei/cat-10234-so.jpg","https://www.ykkap.co.jp/products/door/screen/nhm/img/feature02.jpg"YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm×高1795mm] ykk 玄関網戸 勝手口網戸 ドア用網戸 玄関引き戸用網戸 格子 折れ網
楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅805mm
【楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm×高1795mm]【ykk】【玄関網戸】【勝手口網戸】【ドア用網戸】【玄関引き戸用網戸】【格子】【折れ網戸】: ノース&ウエスト
YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm×高1795mm
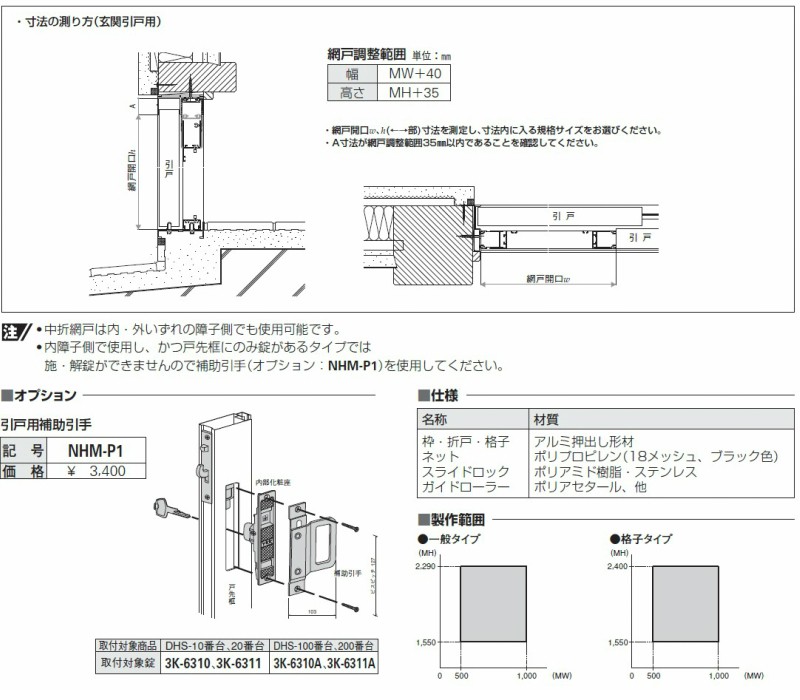
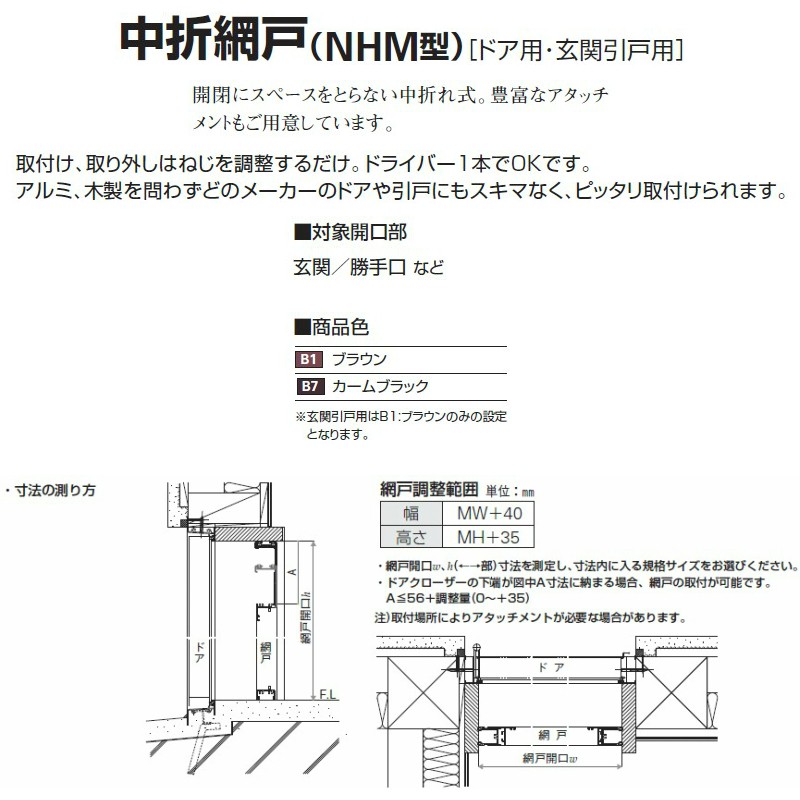
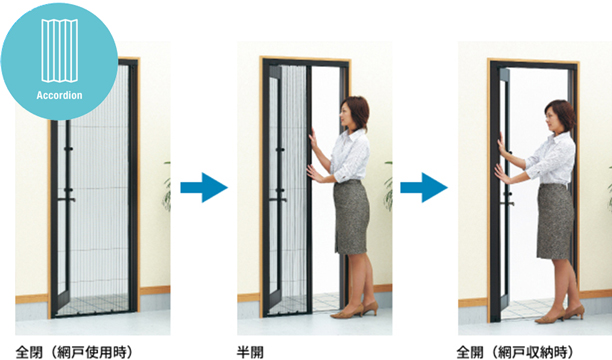
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅875mm×高2300mm
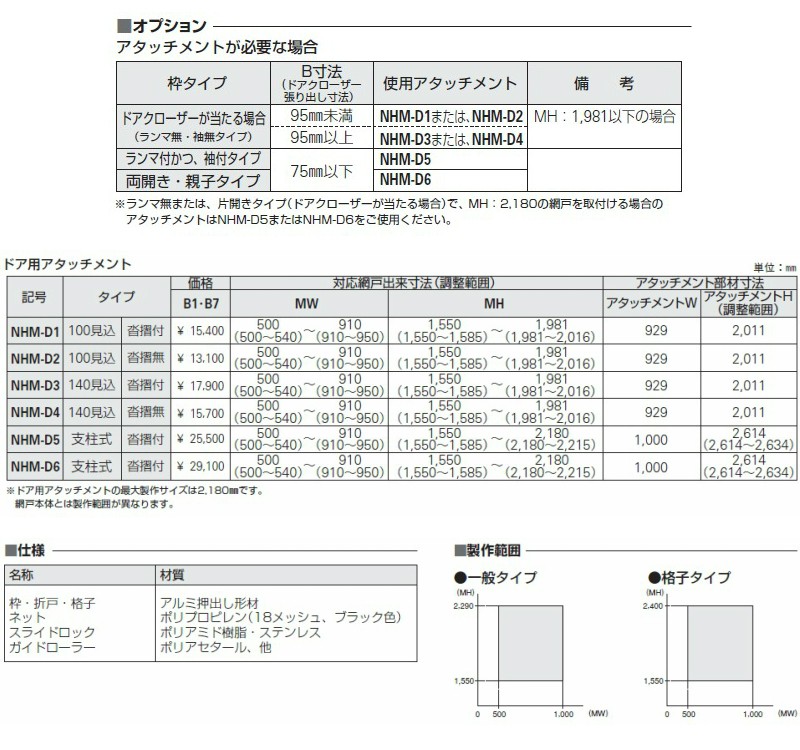
ドア・玄関引戸(引き戸)用 中折網戸 NHM型 ドア用 格子タイプ MW700
ドア・玄関引戸(引き戸)用 中折網戸 NHM型 ドア用 一般タイプ MW805
楽天市場】ykk 中折網戸の通販
YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅1000mm×高2300mm
ノース&ウエスト - YKKAP汎用網戸|Yahoo!ショッピング
中折れ網戸 YKKap 玄関用 - その他
中折れ網戸 YKKap 玄関用 - その他
YKKAP汎用網戸 横引き収納網戸フラットタイプXMA 片引きタイプ 木調色:[幅1300mm×高2330mm] ykk 玄関アミ戸 勝手口アミ戸 ドア用網戸 玄関引戸用
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
玄関 網戸 ykkの人気商品・通販・価格比較 - 価格.com
中折れ網戸 YKKap 玄関用 - その他
中折れ網戸 YKKap 玄関用 - その他
YKKAP汎用網戸 横引きロール網戸フラットタイプXMD 片引きタイプ
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅1000mm×高2300mm
中折れ網戸 YKKap 玄関用 - その他
楽天市場】玄関 網戸の通販
窓 サッシ 玄関網戸 引き戸用の人気商品・通販・価格比較 - 価格.com
中折れ網戸 YKKap 玄関用 - その他
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
YKKAP|玄関引戸|中折網戸|通風網戸|北九州市│北九州市の戸建て
楽天市場】玄関網戸 1820の通販
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
玄関網戸 中折の人気商品・通販・価格比較 - 価格.com
YKKAP汎用網戸 中折網戸NHM型 ドア用 一般タイプ:[幅735mm×高1781mm
楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅805mm
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
中折れ網戸 YKKap 玄関用 - その他
リフォーム 玄関ドア YKKap ドアリモ D30 断熱ドア D2仕様 シック C03
玄関 網戸 ykkの人気商品・通販・価格比較 - 価格.com
楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm
YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm×高1795mm] ykk 玄関網戸 勝手口網戸 ドア用網戸 玄関引き戸用網戸 格子 折れ網
楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅805mm
【楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm×高1795mm]【ykk】【玄関網戸】【勝手口網戸】【ドア用網戸】【玄関引き戸用網戸】【格子】【折れ網戸】: ノース&ウエスト
YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm×高1795mm
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅875mm×高2300mm
ドア・玄関引戸(引き戸)用 中折網戸 NHM型 ドア用 格子タイプ MW700
ドア・玄関引戸(引き戸)用 中折網戸 NHM型 ドア用 一般タイプ MW805
楽天市場】ykk 中折網戸の通販
YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅1000mm×高2300mm
ノース&ウエスト - YKKAP汎用網戸|Yahoo!ショッピング
中折れ網戸 YKKap 玄関用 - その他
中折れ網戸 YKKap 玄関用 - その他
YKKAP汎用網戸 横引き収納網戸フラットタイプXMA 片引きタイプ 木調色:[幅1300mm×高2330mm] ykk 玄関アミ戸 勝手口アミ戸 ドア用網戸 玄関引戸用
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
玄関 網戸 ykkの人気商品・通販・価格比較 - 価格.com
中折れ網戸 YKKap 玄関用 - その他
中折れ網戸 YKKap 玄関用 - その他
YKKAP汎用網戸 横引きロール網戸フラットタイプXMD 片引きタイプ
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅1000mm×高2300mm
中折れ網戸 YKKap 玄関用 - その他
楽天市場】玄関 網戸の通販
窓 サッシ 玄関網戸 引き戸用の人気商品・通販・価格比較 - 価格.com
中折れ網戸 YKKap 玄関用 - その他
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
YKKAP|玄関引戸|中折網戸|通風網戸|北九州市│北九州市の戸建て
楽天市場】玄関網戸 1820の通販
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
玄関網戸 中折の人気商品・通販・価格比較 - 価格.com
YKKAP汎用網戸 中折網戸NHM型 ドア用 一般タイプ:[幅735mm×高1781mm
楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 格子タイプ:[幅805mm
中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社
ドア・玄関引戸用 中折網戸 NHM型 ドア用 格子タイプ MW805-910×MH2180
中折れ網戸 YKKap 玄関用 - その他
リフォーム 玄関ドア YKKap ドアリモ D30 断熱ドア D2仕様 シック C03
玄関 網戸 ykkの人気商品・通販・価格比較 - 価格.com
楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm
商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています

![【楽天市場】YKKAP汎用網戸 中折網戸NHM型 引戸用 一般タイプ:[幅770mm×高1795mm]【ykk】【玄関網戸】【勝手口網戸】【ドア用網戸】【玄関引き戸用網戸】【格子】【折れ網戸】: ノース&ウエスト](https://tshop.r10s.jp/hokusei-nw/cabinet/267m-so.jpg)

![中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社](https://www.ykkap.co.jp/products/door/screen/nhm/img/feature02.jpg)
![中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社](https://www.ykkap.co.jp/products/door/screen/nhm/img/main_sp.png)





![中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社](https://www.ykkap.co.jp/products/door/screen/nhm/img/feature07.jpg)




![中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社](https://www.ykkap.co.jp/products/door/screen/nhm/img/feature04.jpg)



![中折網戸 NHM型[ドア用・玄関引戸用]| YKK AP株式会社](https://www.ykkap.co.jp/asset_2021/image/header_pic_002.jpg)